Does rubbing result in both bodies being charged, and if so, by the same or different charges? To answer the question, you have to make an experiment.
Different charges
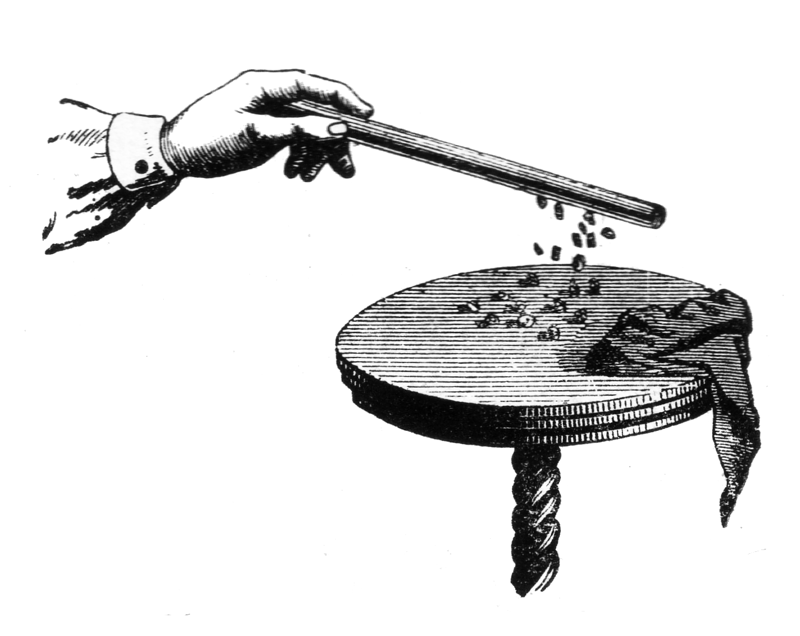
We rub the glass stick with a silk cloth and hold the stick in one hand and the cloth in the other. We take turns approaching the stick and the cloth to the pieces of paper on the table. Both the stick and the cloth pull the pieces of paper towards you. Consequently, both bodies are charged as a result of friction. The question remains whether their charges are the same or different.
This can be checked by placing the glass stick and silk cloth alternately near a charged body (eg an aluminum foil cylinder). When the test is performed, it turns out that when the cylinder is near one body, it pulls and when it is near the other body, it pushes. This means that the bodies that are rubbed against each other receive different charges. When they are measured, it appears that the charges on the bodies are equal. This happens because during friction, the charges of the same name are transferred from one body to another, and in one body there is a lack of these charges and in another a surplus.
During rubbing
- one body receives a negative charge and the other a body a positive charge.
- both bodies receive a negative charge.
- both bodies receive a positive charge.
During rubbing
- both bodies give each other some of their charges.
- gives one body to another part of its charges.
- there is no movement of charges.
Think!
- What happens to charges when the body is electrified by rubbing it with another body?
- Why is there no charge when two bodies of the same material are being rubbed?
Free charges
You have now learned quite a lot about the electrification of bodies, but we did not see the electric charges and their movement in the experiments we performed. But the test results can be explained by differently named charges and their movements. Unfortunately, not all the secrets of nature can be solved with such simple experiments. We also need to trust scientists and what they have found out.
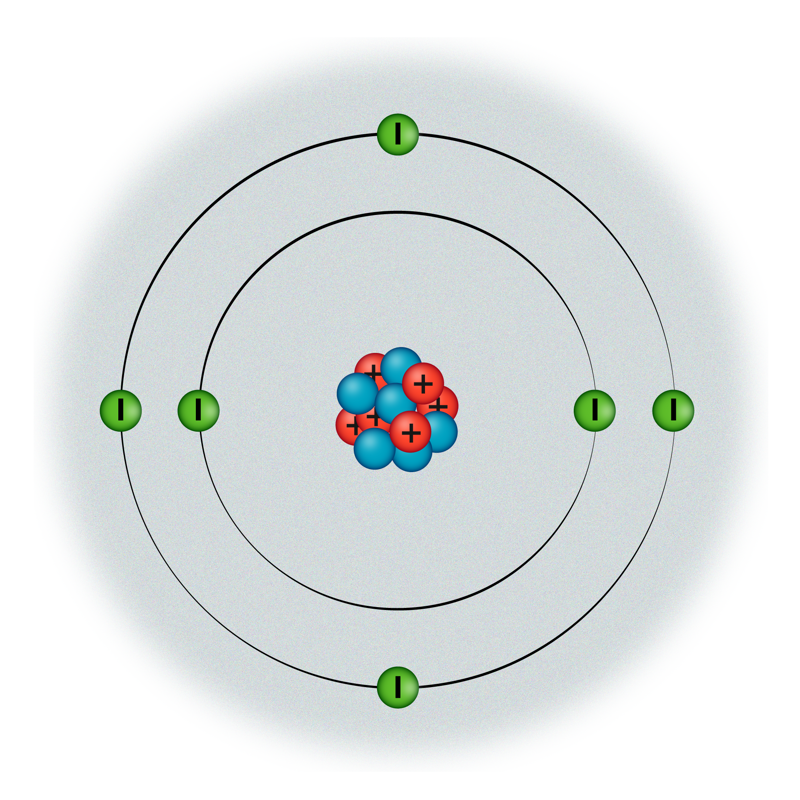
All substances have been found to contain both positively and negatively charged particles. Positively charged particles are protons and they are located in the nucleus of an atom, from which they cannot escape or move freely in matter. Negatively charged particles are electrons, they move around the nucleus in the atom, where they are held by the pull of the positive charge of protons. Various models have been developed to describe the atom, which are described in more detail in Chapter 9.1. So far, we use the atomic planetary model in the explanations.
Electrons can also leave their atom and start wandering around matter. Such electrons are called free electrons. Since the carriers of a positive charge cannot move freely in matter, all phenomena in which the movement of charges occurs must be explained only by electrons.
- Cannot move freely in matter
- Positive charge
- Can't leave the atom
- Negative charge
- Moves around the atomic nucleus
- Located in the atomic nucleus
- Can leave the atom
- Can move freely
Think!
- How do you think free electrons are related to the generation of electric current?
Charge transfer
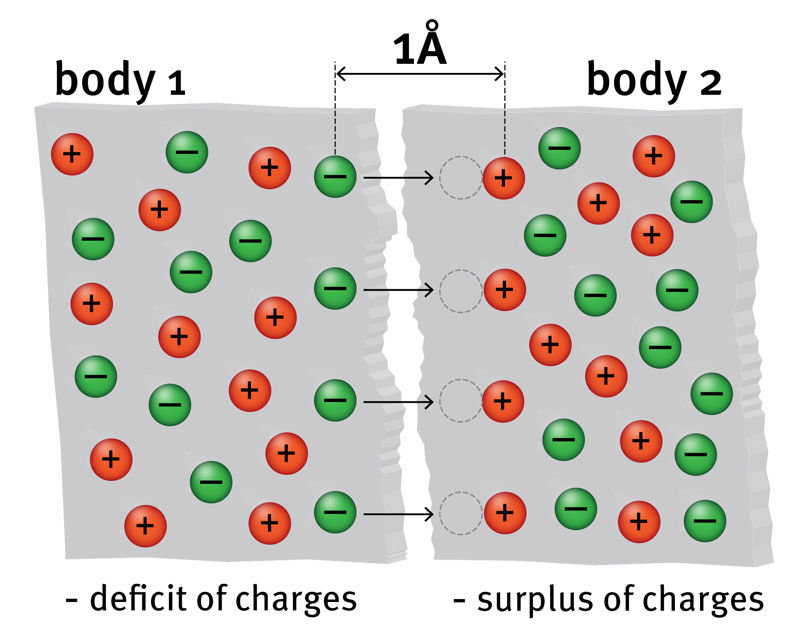
In order for an electron to move from one matter to another, it must be subjected to a force. Such a force can only be an electrical attraction that can be exerted by a proton in one of the atoms of another substance. Accurate studies show that an electron is affected by a significant proton pulling force when the distance of the electron from the proton is less than 10-10 m, ie 1 angstrom (1 Å).
The surface of the body is never completely smooth, there are always bumps that cannot be seen with the naked eye. Therefore, it is not possible to bring bodies so close together that they can transfer electrons from one body to another by simple compression. However, when the bodies are rubbed against each other, there are places where the distance is small enough for the electrons to pass. And since the speed of free electrons is high, this contact is sufficient for the time required for the transition.
In order for anto move from one body to another, it must be subjected to anexerted on it by ain another body. Sufficient force for transmission occurs when the particle spacing isthan 10-10 m. This size is called.
Think!
- What is the name of an atom that has lost an electron?
- Do you think that the same body is always charged with the same charge as a result of friction, for example positively?
Elementary charge
The proton and electron charges are of equal size, and no smaller electric charge has been found on any of the free particles. Therefore, this charge is called an elementary charge. The electric charge of charged bodies is an integer multiple of the elementary charge.
There are always the same number of protons and electrons in the uncharged body. Since both the proton and electron charges are exactly equal but opposite, the total charge in the body is zero. As the electrons of frictional bodies move from one body to another, the body that donated the electrons charges positively and the other body charges negatively. This is because the law of charge persistence applies: no electrical charges are created or lost.
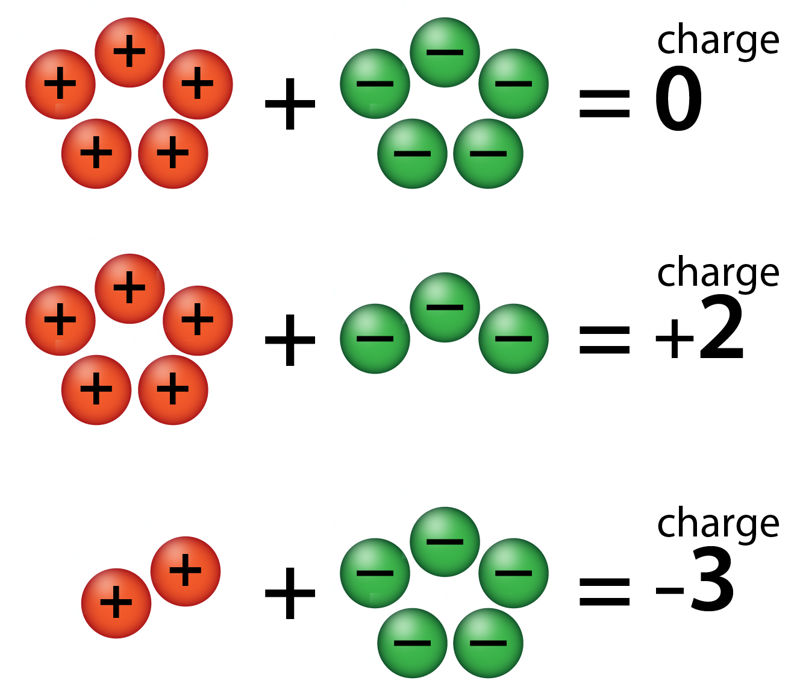
(+2) + (−2) =
(+2) + (−1) =
(+1) + (−2) =
(−5) + (+3) =
(−4) + (+6) =
Unit of electric charge
The unit of electric charge is 1 coulomb (1 C), named after the French physicist Charles Augustin de Coulomb. He discovered the law of interaction of electrified bodies. One coulomb has a very large charge. For example, by rubbing a glass rod, we can never give it a charge greater than one microcoulomb (1 µC). However, the elementary charge is still much smaller, its value is 1.6 ∙ 10-19 C.

Think!
How many protons should there be in the body for the body charge to be 1 coulomb?
Grounding

We have learned to charge the bodies, but what if we want to get rid of the charge? The first idea may be that we charge the body with the opposite charge and then the total charge is zero. But how could we choose the right amount of opposite charge? When the charge is low, it is not enough to reset the existing charge, but when there is more, the body charges in the opposite direction.
The surest way to lose a charge is to connect a charged body with a metal wire to some very large uncharged body or Earth. It is said that the body is grounded. In this case, the electric charge moves from the charged body to another body whose charge does not change significantly. For example, there are a huge number of both positive and negative charges in the earth, and the charge from our test body does not change the total charge on Earth. The situation can be compared to emptying an inflated balloon. The air in the balloon is compressed, so there is more of it in the same volume than elsewhere. When we open the ball, air flows out of it. Does the air coming from the balloon increase the air pressure in the room? No, because it is very small compared to other air. In this way, the Earth's electric charge does not change when the charge of our test body is transferred to it. Now we can also justify why the hand must be gloved when electrifying metal, ie why the hand must be isolated from the body to be charged. If this were not the case, the charge would move from the metal as a very good conductor to our body and the charge from the metal would remain zero.
During grounding, the electric charge of the charged body travels to another body with a metal rod or wire, the dimensions of which are than the dimensions of the charged body.
For groundingis used often.
Find out!
What is lightning protection and how does it work?
I'll remember
- The protons in the atomic nucleus are positively charged.
- Electrons have a negative charge and can be both atomic and free.
- The charges of a proton and an electron are exactly equal, and this charge is called an elementary charge.
- The electric charge of a charged body is an integer multiple of an elementary charge.
- During the rubbing of two bodies, the bodies get very close to each other in some places and electrons can move from one body to another.
- The body that gives away electrons charges positively, and the body that receives electrons charges negatively.
- There are always the same number of protons and electrons in an uncharged body.
- The charge that the body receives during electrification can be transferred to Earth, or grounded, by means of a metal wire.