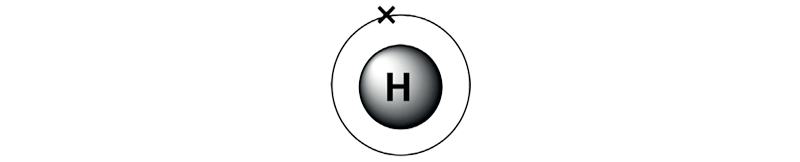
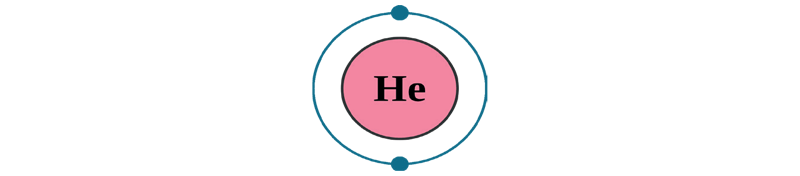
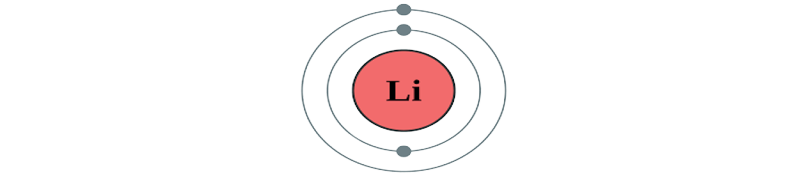
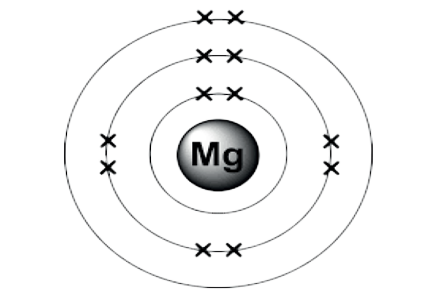
All substances that occupy space and have mass are known as matter. All matter is made by combining many tiny particles.
Think and Talk
- In Grade 8 you learnt about atoms and elements. What is the meaning of atoms and elements?
- What is found inside an atom?
Discover
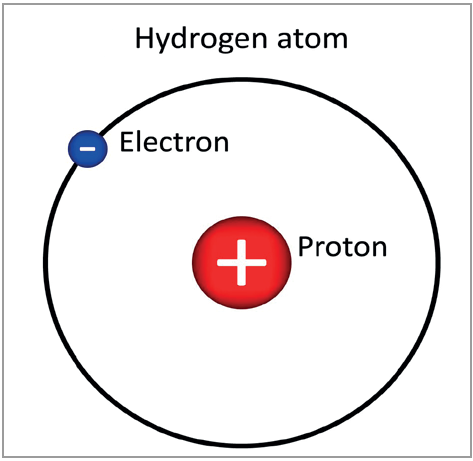
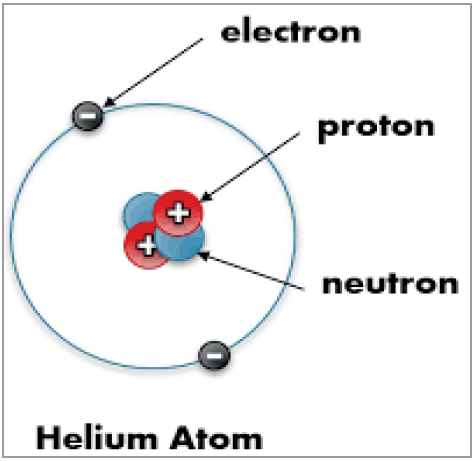
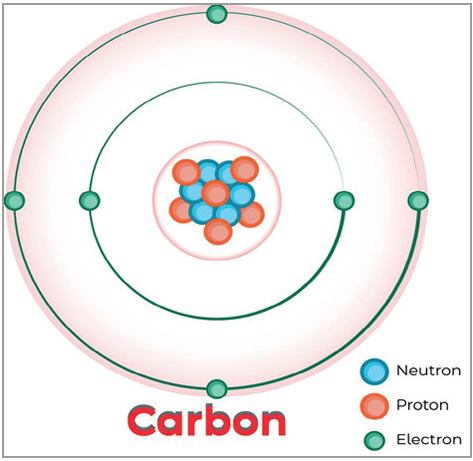
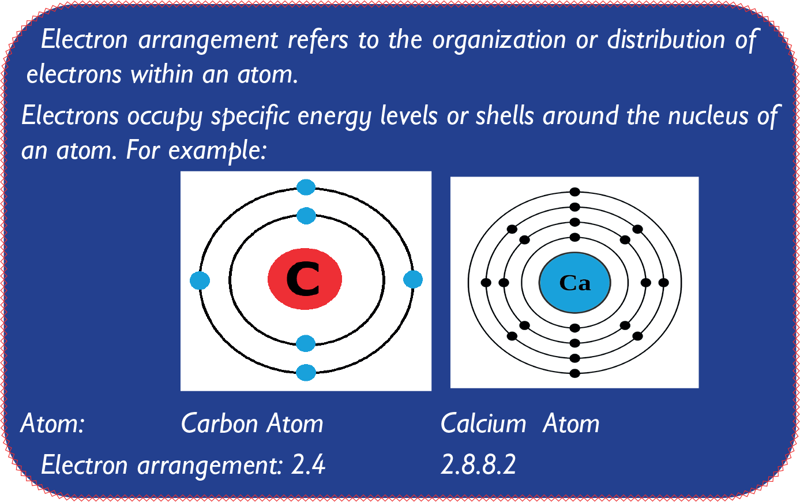
- An atom is the smallest unit of an element.
- All matter, such as gases like hydrogen and carbon and solids like carbon, have their atoms having different combinations of the same three components.