Properties of matter in different states
In previous grades, you were introduced to matter and the different states of matter. You are now going to review what you learnt and explore more about matter.
Activity 1.1: Reviewing learnt ideas about matter
Work in groups
Requirements: Manilla paper, Internet access and reference books.
Procedure:
Discuss and answer the following questions and prompts.
- What is matter?
- How does matter exist?
- Draw a flow chart to show the relationship between the different states of matter.
- Search the Internet for videos and animations to gather more information on matter.
- Present your findings to the rest of the learners in your class.
Points to note
In daily life, we encounter different substances also called matter. The air we breathe, the water we drink and the soil we walk on are all forms of matter. Matter is anything that has mass and occupies space. Matter exists in three states, namely solid, liquid and gas (vapour/air).
The three states of matter can be changed from one state to another, as shown in Figure 1.1.
Matter can be changed from one state to another via heating, cooling, or pressure changes.

Physical properties of matter
Matter is composed of very tiny particles that exist together in three states: solid, liquid and gas. Each of the three states of matter have specific properties, which are determined by the way these particles are arranged, as shown in Figure 1.2.
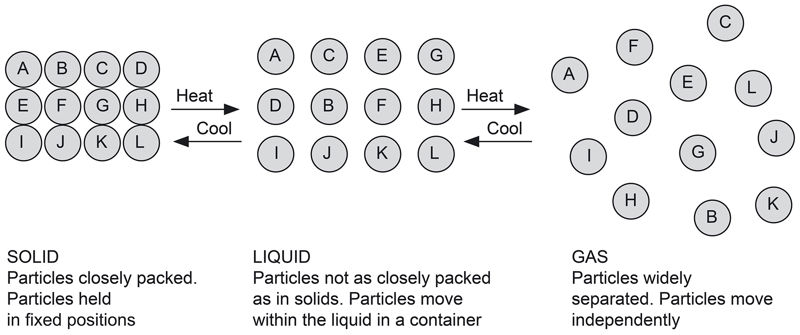
In the following activities, we shall investigate the physical properties of the three states of matter.
Activity 1.2: Investigating the physical properties of solids
Work in groups
Requirements: A piece of stone, a glass prism, a graduated 250 ml beaker, a piece of sewing thread and a weighing balance.
Procedure:


1. Examine the appearance of the solid provided. See Figure 1.3.
What can you say about the form and appearance of the solid?
2. Squeeze the solid with your fingers. See Figure 1.4.
Is it possible to change the shape of the solid easily? Explain why or why not.
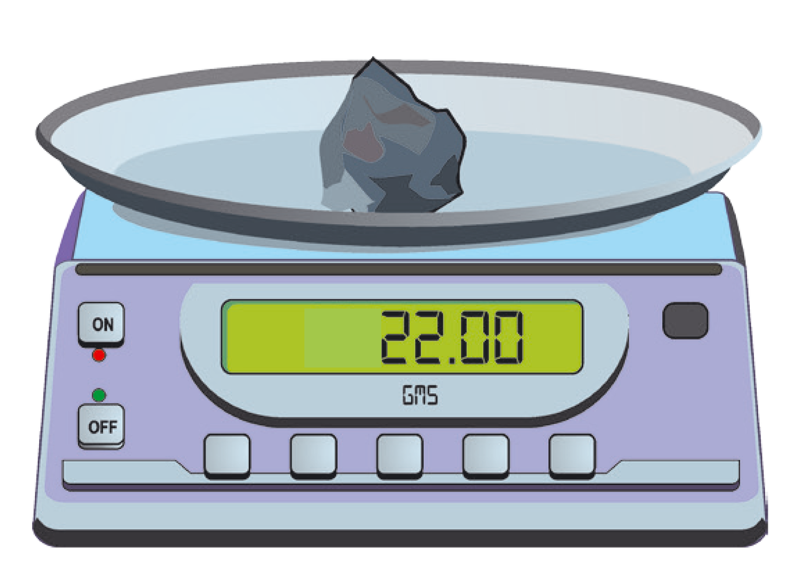
3. Using a weighing balance, determine the mass of the solid. See Figure 1.5.
Record the mass: grams
4. Pour 200 ml of water into a beaker. Note and record the level of the water in the beaker (V1).
5. Tie the stone with a piece of thread and carefully lower it into the beaker. See Figure 1.6.

6. Record the new volume of water in the beaker (V2).
V1:
V2:
The difference in water level:
What can you say about the change in water level?
7. Repeat procedures 1 to 6 with the glass prism.
The mass of solid: grams
V1:
V2:
The difference in water level:
1. What can you say about the masses of the different solids used?
2. Explain and compare the differences in water levels when each solid is dipped in the water.
3. Using the mass obtained in procedure 4 and the volume obtained in procedure 1, discuss the density of the solids used.
Points to note
The piece of stone and glass prism represent matter in the solid state. Solids have definite shapes and are not compressible. This is because the particles in a solid state are already closely packed together and held in fixed positions. For this reason, it is not easy to change the shape of a pure solid by squeezing.
Mass is a measure of the amount of matter in a substance. Different amounts of the same substance contain different amounts of matter, and therefore have different masses.
Volume is the space occupied by a substance. When the solids are put in water, the water level rises. The volume of the solid is represented by the displacement of the water.
Volume of solid = V2 – V1
Volume of regular solid = L × W × H
From the mass and volume of solids, the density (amount of matter per given volume) can be calculated. Density is mass per unit volume, it can be calculated using the following formula:
Activity 1.3: Investigating the physical properties of liquids
Work in groups
Requirements: A 250 ml beaker, a weighing balance, a 250 ml measuring cylinder, a 250 ml round bottomed flask, a 250 ml conical flask, a water trough, a 100 ml syringe and water.
Procedure:
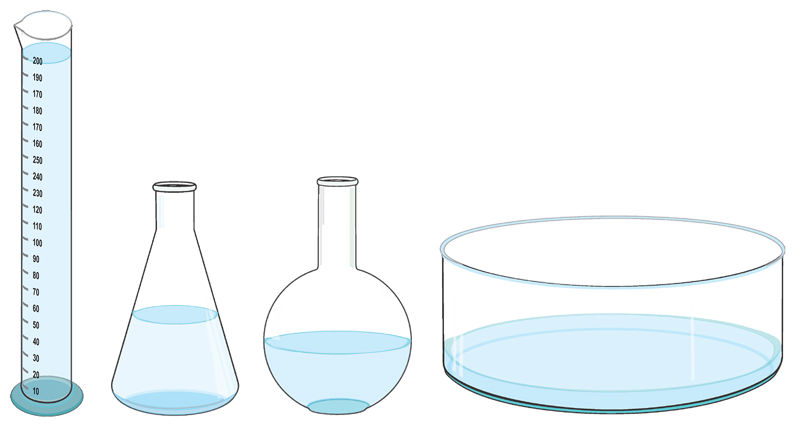
1. Place 200 cm3 of water into a 250 ml measuring cylinder. Note the shape of the water in the cylinder.
2. Transfer the same volume of water from the cylinder to the following containers successively:
- 250 ml conical flask
- 250 ml round bottomed flask
- water through.
a) Describe the shape of the water in each of the containers used.
b) Comment on the shape of the water in the different containers.
c) Comment on the volume of the water in the different containers.
d) Explain why it is possible to transfer water from one container to another.
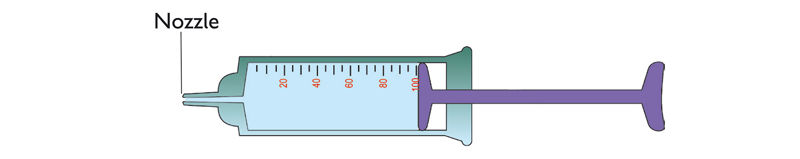
3. Fill a 100 ml syringe with water as shown in Figure 1.7.
4. Block the nozzle of the syringe with your thumb. Push the piston of the syringe in.
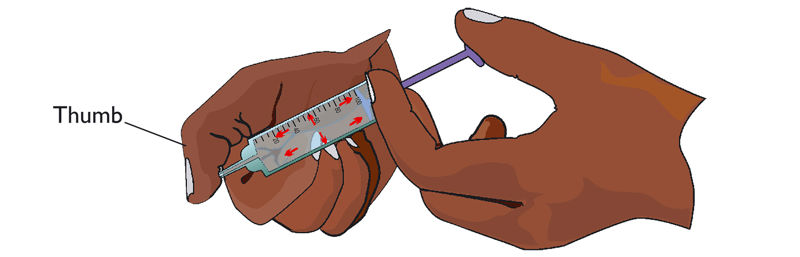
Explain why it is not easy to push the piston of the syringe filled with water.
5. Weigh a 250 ml beaker and record its mass: grams.
6. Add 200 ml of water into the beaker and weigh it again. Record the mass: grams.
What is the mass of the water in the beaker? grams
7. Colour 25 ml of water and transfer it to a 250 ml beaker. Add 25 ml of kerosene, shake the mixture and allow it to settle.

Explain what you observe when coloured water and kerosene are mixed and allowed to settle.
Points to note
When 200 ml of water is transferred into containers of different shapes, the water settles and takes on the shape of the container. The volume, however, remains the same.
It is possible to transfer water (a liquid) from one container to another by pouring, because water flows. When a liquid is poured into containers of different shapes, it flows and takes on the shape of the new container, as shown in Figure 1.10.
Liquids are not easily compressed. This explains why it is not possible to push the piston with the outlet of the syringe blocked.
Liquids have a definite mass and volume, but have no definite shape; they take up the shape of the containers in which they are placed.
When a beaker is weighed before and after adding 200 ml of water, there is a notable increase in mass. The increase in mass represents the mass of the water that has been poured into the beaker. This indicates that liquids have mass.
When water and kerosene are mixed, the mixture is not homogeneous. They are said to be immiscible. Kerosene floats on the water, as shown in Figure 1.11. This is because the density of kerosene is lower than that of water. When immiscible liquids are mixed, the liquid with a lower density floats on top of the heavier one.

Activity 1.4: Investigating the physical properties of gases
Work in groups
Requirements: Two balloons, a half meter rule, a syringe, four gas jars, bromine liquid, a dropper, vaseline jelly and a timer or stopwatch.

Procedure:
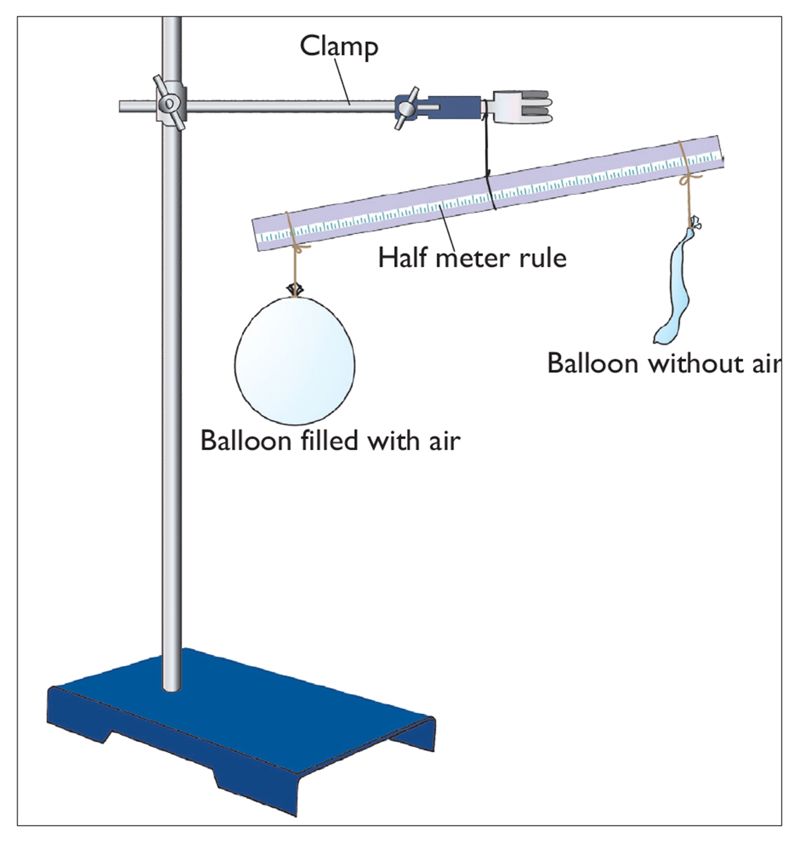
1. Balance two empty balloons as shown in Figure 1.12.
2. Remove one balloon and inflate it.
3. Tie the inflated balloon back on the same spot as before. What do you observe when the inflated balloon is tied back at the same spot?
4. Draw air into a syringe and block its nozzle.
5. Gently push the piston as far as it can go without letting any air escape, as shown in Figure 1.13.
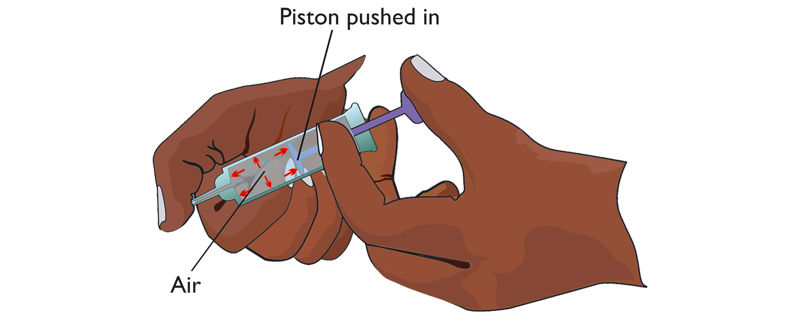
What is observed when the piston of the syringe is pushed in?
6. While still blocking the nozzle, release the piston.
What is observed when the piston of the syringe is released? Record your observation.
 | Bromine is poisonous. The next experiment should be done in a fume chamber or in an open space by the teacher. |
7. Place a drop of bromine liquid carefully at the bottom of a gas jar using a dropper, and cover the jar with a gas jar lid coated with vaseline jelly. Allow the jar to stand for five minutes. Record your observations.
8. Invert a gas jar containing bromine over a gas jar containing air as shown in Figure 1.14(a).

Explain your observation after two minutes.
9. Repeat procedures 7 and 8, but now invert a gas jar of air over the one containing bromine. See Figure 1.14(b).
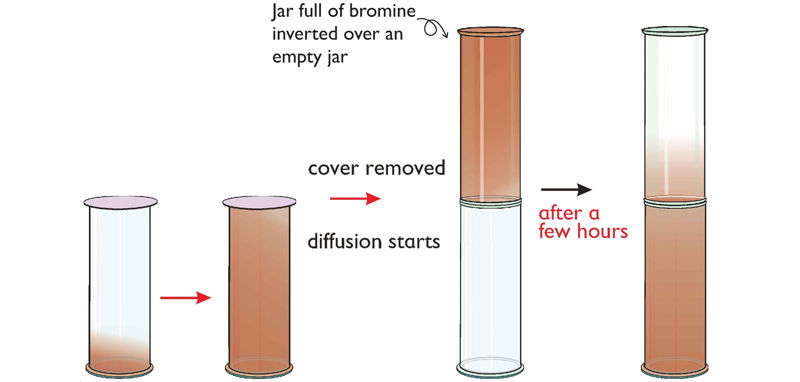
Record your observation after two minutes.
Explain your observations of the gas jars in procedure 8 and 9.
Points to note
Initially, when the two balloons were empty, the beam was horizontal. After inflating one balloon, the beam balance tilted, as shown in Figure 1.15. This shows that the inflated balloon is heavier than the empty one due to the air contained inside.
When a fixed amount of air is enclosed inside a syringe and the piston is pushed, the piston moves until it can no longer move. The volume of air in the syringe decreases.
When the piston is released, the air in the syringe pushes out the piston to occupy a larger space, as in Figure 1.16. The experiment shows that air can be compressed by the application of pressure. Therefore, gases can be compressed to occupy a smaller volume and, if allowed, gases can spread to occupy a large space.
The observation shows that gases have a definite mass, but do not have a definite volume or shape.
Bromine is a highly volatile liquid. When a few drops of bromine liquid are placed in a gas jar, it quickly turns into vapour and spreads, filling the gas jar. When the gas jar containing bromine is inverted over the gas jar of air, bromine vapour quickly moves down into the lower gas jar. However, when the gas jar containing air is inverted over the gas jar of bromine, the brown bromine vapour takes a long time to move to the upper jar. This demonstrates that matter in the gaseous state has different densities. Bromine, being denser than air, quickly spreads/diffuses to the lower jar, but takes longer to diffuse into the upper jar.

The following is a summary of some of the physical properties of matter in the solid, liquid and gaseous state.
Table 1.1: Physical properties of solids, liquids and gases
Solids | Liquids | Gases |
Have a definite shape. | Have no definite shape; they take on the shape of the container. | Have no fixed shape; they occupy the entire space they are enclosed in. |
Have a fixed volume. | Have a fixed volume. | Have no fixed volume. |
Do not flow. | Flow easily, as particles slide over each other. | Flow easily, as particles collide with each other while they move randomly. |
Cannot be compressed. | Are not easily compressed. | Are easily compressed. |
Have specific densities. | Have specific densities. | Have specific densities. |
